

Quickvue at home Otc Covid 19 Test Kit in South Korea
1.20 - 1.50 USD ($)/Box
Product Details:
X
Quickvue at home Otc Covid 19 Test Kit in South Korea Price And Quantity
- 1 Box
- 1.20 - 1.50 USD ($)/Box
Quickvue at home Otc Covid 19 Test Kit in South Korea Trade Information
- USA
- 3 Box Per Day
- 5-15 Days
- North America South America Eastern Europe Western Europe Middle East Central America Asia Africa
- Quickvue at-home otc covid-19 test kit in South Korea.FDA EUA CE ISO GMP SFC ETC
Product Description
Feature
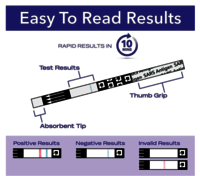
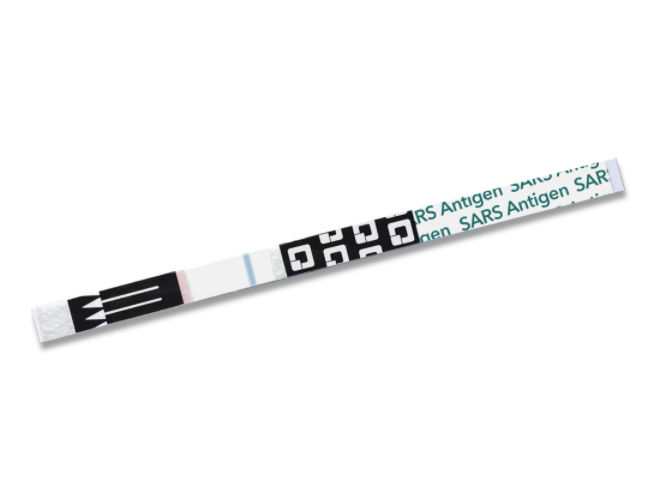
- The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid proteins from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests.Quickvue at-home otc covid-19 test kit in South Korea
- For Use Under an Emergency Use Authorization (EUA)
- This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal(NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older
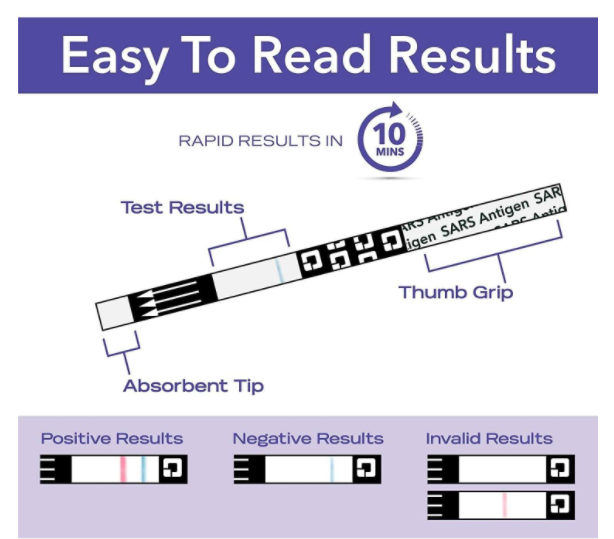
- Positive results indicate the presence of viral agents, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite cause of disease. Individuals who test positive with the QuickVue At-Home OTC COVID-19 Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary
- Negative results should be treated as presumptive,Quickvue at-home otc covid-19 test kit in South Korea do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
- Negative results should be considered in the context of an individual recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management
- Individuals should provide all results obtained with this product to their healthcare provider for public health reporting
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens
- The test is intended to be read at 10 minutes; If the test is read before this or is read more than 5 minutes after the indicated read time, results may be inaccurate and the test should be repeated
Other Products in 'AT -home covid-19 test in Different countries' category
Get in touch with us